FoodNet International Holdings Pte. Ltd. is a one stop solution provider for the food industry and has its headquarters in Singapore
FoodNet International Holdings Pte. Ltd. is a one stop solution provider for the food industry and has its headquarters in Singapore

CORPORATE OVERVIEW
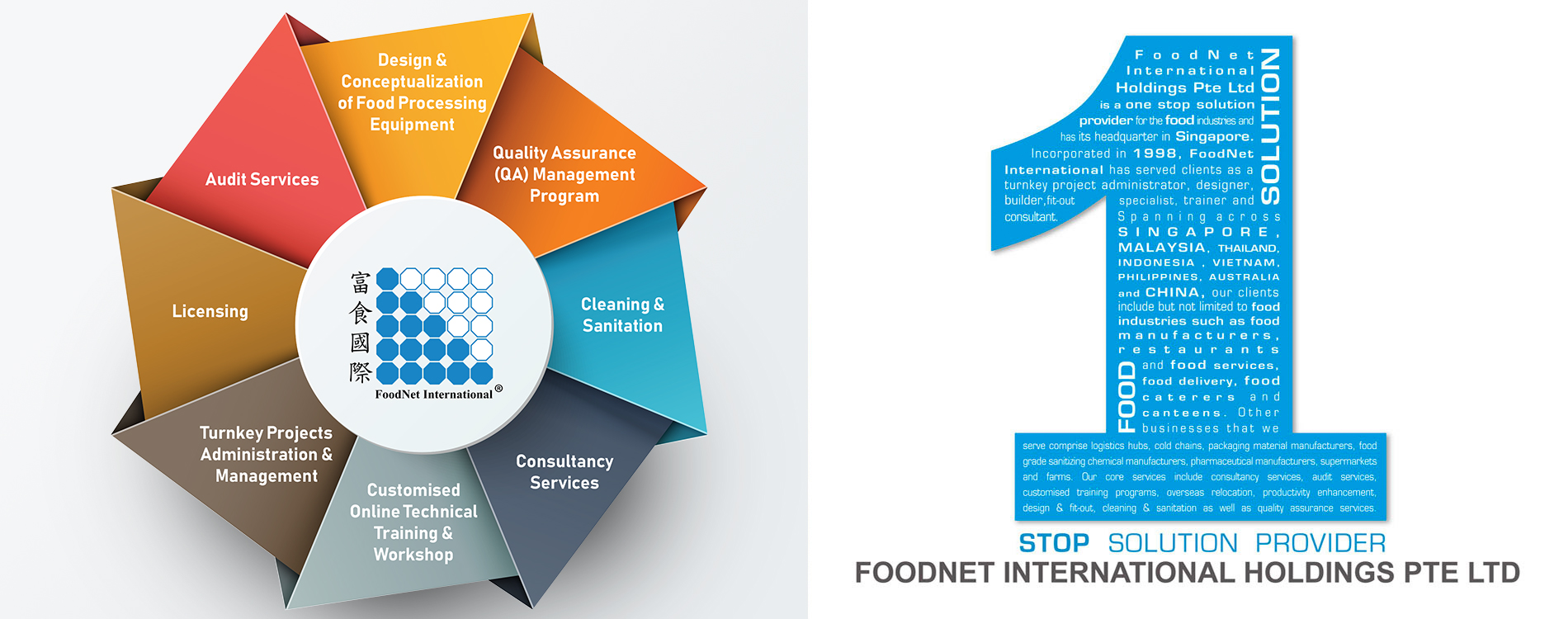
FoodNet International Holdings Pte. Ltd. is a one stop solution provider for the food industries and has its headquarters in Singapore. Incorporated in 1998, FoodNet International has served over 1,200 clients as a turnkey project administrator, designer, builder, fit-out specialist, trainer and consultant. Spanning across Singapore, Malaysia, Thailand, Indonesia, Vietnam, Philippines, Australia and China, our clients include but not limited to food industries such as food manufacturers, restaurants and food services, food delivery, food caterers and canteens. Other businesses that we serve comprise logistics hubs, cold chains, packaging material manufacturers, food grade sanitizing chemical manufacturers, pharmaceutical manufacturers, supermarkets and farms.

CORPORATE OVERVIEW
FoodNet International Holdings Pte. Ltd. is a one stop solution provider for the food industries and has its headquarters in Singapore. Incorporated in 1998, FoodNet International has served over 1,200 clients as a turnkey project administrator, designer, builder, fit-out specialist, trainer and consultant. Spanning across Singapore, Malaysia, Thailand, Indonesia, Vietnam, Philippines, Australia and China, our clients include but not limited to food industries such as food manufacturers, restaurants and food services, food delivery, food caterers and canteens. Other businesses that we serve comprise logistics hubs, cold chains, packaging material manufacturers, food grade sanitizing chemical manufacturers, pharmaceutical manufacturers, supermarkets and farms.
FOUNDER MESSAGE
Food is a vital part of human existence. Food serves not only as a need, it is also a source of pleasure and communion. As such, extra precaution needs to be taken to ensure that the food that is served is fit for consumption and devoid of contamination. Food sources have to be fresh and clean, while preparation processes have to be safe and hygienic for daily consumption.
With the above vision in mind, FoodNet International Holdings was set up in 1998 with the mission to ensure that safe foods are prepared through proper sanitization and the highest degree of hygiene.
FOUNDER MESSAGE
Food is a vital part of human existence. Food serves not only as a need, it is also a source of pleasure and communion. As such, extra precaution needs to be taken to ensure that the food that is served is fit for consumption and devoid of contamination. Food sources have to be fresh and clean, while preparation processes have to be safe and hygienic for daily consumption.
With the above vision in mind, FoodNet International Holdings was set up in 1998 with the mission to ensure that safe foods are prepared through proper sanitization and the highest degree of hygiene.
OUR CORPORATE CAPABILITIES
OUR CORPORATE CAPABILITIES

CONSULTANCY SERVICES
- Food Safety Management System
- Quality Management System
- Environmental Management System
- Occupational Health and Safety Management System
- Good Distribution Practice for Medical Devices
- Good Manufacturing Practices for Cosmetic Products,
Food Industry & Pharmaceutical
- Food Safety System Continuous Improvement Program
- Good Hygiene Practices Program for Food Industry
- Development of Food Service Concepts and Integrate with Central Processing Facilities
- Central Kitchen/Plant Design and Layout
- Development of Cleaning and Sanitation Programs
AUDIT SERVICES
- Supplier Assessment Program (SAP) and Audit
- Second Party Food Safety Audit
- Calibration Service for Chillers and Freezers


TURNKEY PROJECTS ADMINISTRATION AND MANAGEMENT (LOCAL AND INTERNATIONAL)
- “Design and Fit Out” for Food Related Premises
- “Build-Operate-Transfer” Projects
- Productivity Enhancement Program
- Risk Assessment and Management
– Workplace Safety and Health - Overseas Relocation for Food Handling Premises
i-CARE SERIES OF TECHNICAL TRAINING PROGRAM AND WORKSHOP
PROFESSIONAL QUALITY
ASSURANCE SERVICES


DESIGN AND CONCEPTUALISE OF FOOD PROCESSING EQUIPMENT
PARTNERS IN IMPROVEMENT

OUR GROUP OF COMPANIES
FoodNet International Holdings Pte. Ltd. comprises of the following companies.